Why Choose a Copper Distiller:
- The Superiority of Copper Over Stainless Steel
Stainless steel has only one advantage over copper: it is cheaper. Traditional distilleries do not use steel alembics, nor do artisanal micro-distilleries and whisky distilleries. They cannot afford a metallic aftertaste, and the shape of the alembics is patented and treated as a valuable secret of each distillery.
- Maintenance and Cleaning
Copper equipment, paradoxically, requires less frequent cleaning than steel equipment with a copper catalyst, which must be etched, neutralized, dried, and refilled after each process. Copper distillers only need to be rinsed with water every few processes, and in case of persistent dirt, "backwash water," the water remaining in the tank after the process, can be used for cleaning. Its slightly acidic properties clean even the toughest dirt.
- Sulfur Compounds
During fermentation, sulfur-containing compounds appear in the mash and wash. Copper absorbs these compounds on its surface, preventing them from entering the distillate, thus eliminating the "eggy" smell and the repulsive aroma of hydrogen sulfide. Copper catalyzes the breakdown of esters and other volatile substances formed during fermentation, which could spoil the taste of the distillate if they penetrate it.
- Ethyl Carbamate
Alongside sulfur, a number of other undesirable components are produced during fermentation. One of these is carcinogenic ethyl carbamate, which is absorbed and returned to the boiler in copper distillers, whereas it entirely passes into the distillate in copper-free equipment (glass, stainless steel). Only copper equipment ensures distillate free from this highly harmful compound!
- Heat Conduction
Copper conducts heat much better, requiring less energy for the process and ensuring more stable rectification. This also means less water is used for cooling compared to other materials, leading to significant savings.
- Special Heating Elements
Our equipment is equipped with special ULWD (Ultra Low Watt Density) heating elements. The power per unit surface area of the heater is so low (below 7W/cm²) that our heaters allow even thicker mashes to be boiled without the need for clarification or filtering. You can boil wines with fruits, seeds, or leftovers from tinctures without worrying about burning or damaging the heater. Therefore, screw-in heating elements, like those for water boilers with powers often 2x2000W or even 3x2000W concentrated on a few cm², are unsuitable for distillation! They are good for boiling water but not for anything thicker and categorically unsuitable for distillation.
- Longevity and Taste
Copper equipment lasts for decades, does not corrode, and does not introduce rust and metallic taste into the distillate, which is common in steel installations.
- Why Not Copper Tanks?
You might ask, "Why don't you use copper tanks?" Oh. we do! Please note however, that a copper tank is dissolved by acidic mashes and washes (and yeast likes an acidic environment), so it wouldn't be as durable as a steel tank. Of course, the vapors coming out of it are harmless to the column. Moreover, unlike columns, copper tanks need to be cleaned after every process. In large alembics at distilleries, this is not a problem—an employee can enter with a brush and clean every nook and cranny. In small tanks, we always ensure the fitting to be large enough for easy cleaning!
- Bacteriostatic Properties
Copper is bacteriostatic—bacteria will not develop in an unused column. Recent studies show that even very dangerous strains like E. coli O157 die within just a few hours on a copper surface, even in dry conditions. The same bacteria survive over a month on a stainless steel surface...
- Influence on Taste
Copper affects the product's taste, making it much more delicate than from steel equipment. Molecular-level reactions between ethanol and copper have been proven. Here is a taste and smell comparison graph of the same distillate from copper and steel equipment:
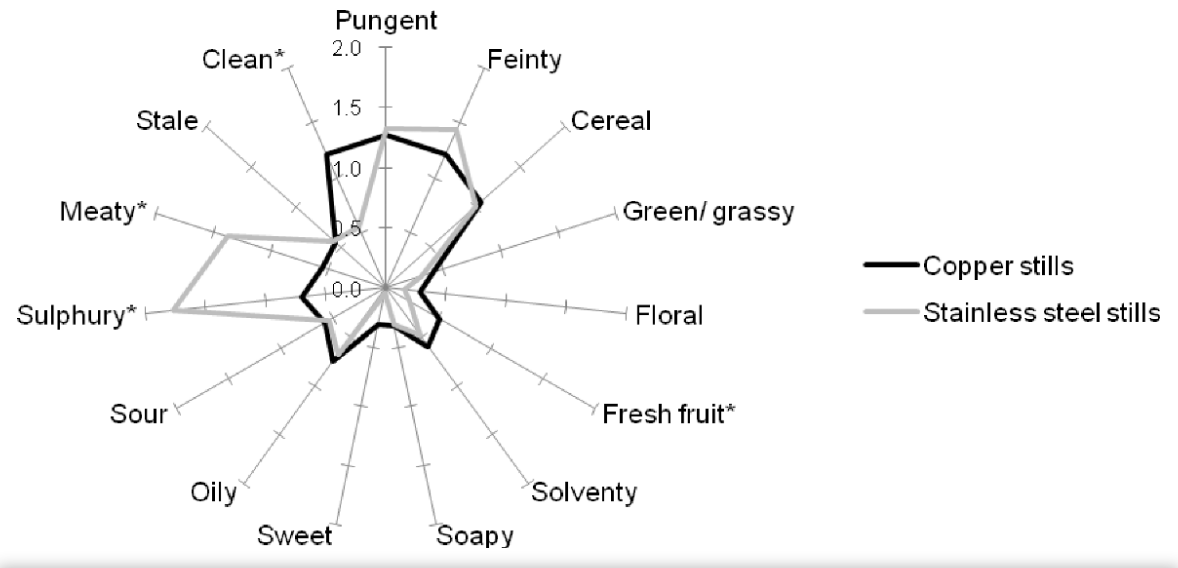
Scientific Foundations
The above information is not without scientific basis. Here are sources where you can read much more about the advantages of copper:
Alcarde A et al. Ethyl carbamate kinetics in double distillation of sugar cane spirit. J Inst Brew 2012;118;352-5
Broom D. World atlas of whisky. Mitchell Beazley 2010
Bryce JH et al (ed). Distilled spirits: Production, technology and innovation. Nottingham Univ Press 2008
Forbes, RJ. Short history of the art of distillation. Brill 1948
Harrison, B et al. The Impact of Copper in Different Parts of Malt Whisky Pot Stills on New Make Spirit Composition and Aroma. J Inst Brew 2011;117(1);106-112
Hernández-Gómez L et al. Melon fruit distillates. Food Chem 2003;82;539-543
Jack, FR et al. Sensory implications of modifying distillation practice in Scotch malt whisky production. In Distilled Spirits, ed Bryce JH, Piggott JR, Stewart GG. Nottingham Univ Press 2008.
Lima U et al. Influence of fast and slow distillation on ethyl carbamate content and on coefficient of non-alcohol components in Brazilian sugarcane spirits. J Inst Brew 2012;118;305-8
Masuda, M and Nishimura, K. Changes in volatile sulfur compounds of whisky during aging. J Food Sci 1982; 47(1); 101-5
Monica Lee KY et al. Origins of flavour in whiskies and a revised flavour wheel. J Inst Brew 2001;107(5);287-313
Morewood S. A philosophical and statistical history of the inventions and customs of ancient and modern nations in the manufacture and use of inebriating liquors. Longman 1838.
Nedjma M, Hoffmann N. Hydrogen sulfide reactivity with thiols in the presence of copper in hydroalcoholic solutions or cognac brandies. J Agric Food Chem 1996;44;3935-38
Nóbrega I et al. Ethyl carbamate in cachaça. Food Chem 2011;127;1243-7
Prado-Ramírez R et al. The role of distillation on the quality of tequila. Int J Food Sci Tech 2005;40;701-8
Reaich, D. Influence of copper on malt whisky character. In Proceedings of 5th Aviemore Conference on malting, brewing & distilling. 1998
Riachi L et al. Review of ethyl carbamate and polycyclic aromatic hydrocarbon contamination risk in cachaça and other Brazilian sugarcane spirits. Food Chem 2014;149;159-169
Russell I. Whisky. Elsevier 2003.
Walker GM, Hughes PS (ed). Distilled spirits, new horizons: energy, environment and enlightenment. Nottingham Univ Press, 2010
